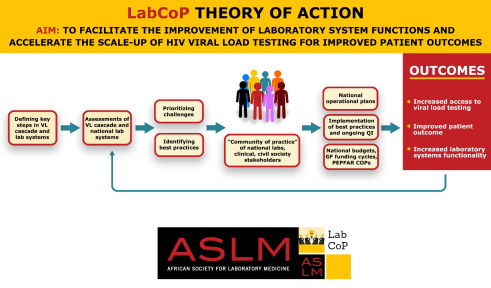
Accurate monitoring of HIV viral load (VL) in a patient is essential for assessing the effectiveness of antiretroviral therapy (ART) and preventing unnecessary switches to costly second-line drug therapy. Unfortunately, VL testing is not widely available due to limited laboratory capacity and cost considerations, so many countries are exploring ways to expand access to this test. This includes investigating more feasible testing and sample collection like dried blood spots (DBS) and point-of-care (POC) technologies, and transport options.
With the continued need for rapid scale-up of ART and an increasing emphasis on VL for ART monitoring, there is an urgent need to expand VL testing capacity within Africa. ASLM recognises that the complexity of scaling-up VL testing is not only a laboratory issue, but it also relates to clinicians and programme managers. Through the Laboratory Systems Strengthening Community of Practice (LabCoP), ASLM provides a venue for sharing information, tools and resources that will support clinical, policy, programmatic, technical and scientific aspects of the implementation of VL testing.